In experiments and clinical settings, catheterize a small vessel for sample collection and drug delivery is quite often. Catheter with a diameter smaller than 1 mm, “microcatheter”, is frequently used. Healthcare personnel and researchers most commonly use syringes with standard Luer connection for fluid collection and drug delivery. Therefore, a Luer connection between catheters and fluid collecting or distributing devices will provide great convenience for secure and reliable operation. However, there are a few problems and unsolved issues in this field.
Common method for connecting a catheter to a standard Luer connector is by modification of the proximal end of a catheter lumen through heat forge. The method for assembling a Luer needle, which is to apply anaerobic glue or epoxy glue, doesn’t work for assembling microcatheters. Anaerobic glue cures when it is sealed off oxygen, which only happens when the two connecting parties fit each other, etc, the smaller one has outer diameter similar the inner diameter of the larger one. This method cannot connect a “micro” tubing to an industry standard Luer connector because their fitting does not provide the needed anaerobic environment. A suboptimal method to circumvent this problem is to use transitional tubing with increased diameter that may provide connection to a Luer needle or a Luer Lock connector. However, its tradeoff is the significantly increased inner volume and a fatty catheter body that is too big to fit into a small vessel. Epoxy glue can be used to assemble a metal Luer needle, but not a microcatheter in non-anaerobic environment. Epoxy produces significant amount of heat while it cures. The tubular body of a microcatheter is made of venerable soft plastic materials. The process of epoxy curing and drying will cause structural damage to the soft plastic tubular body.
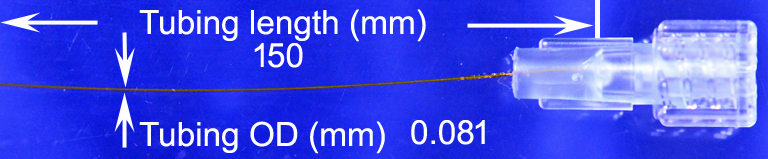
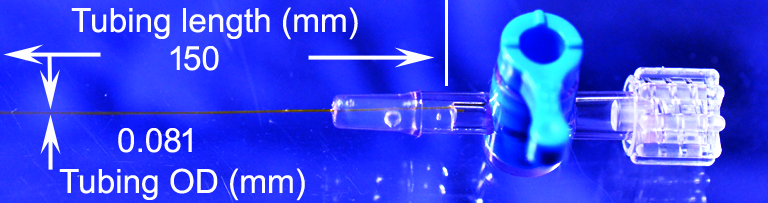
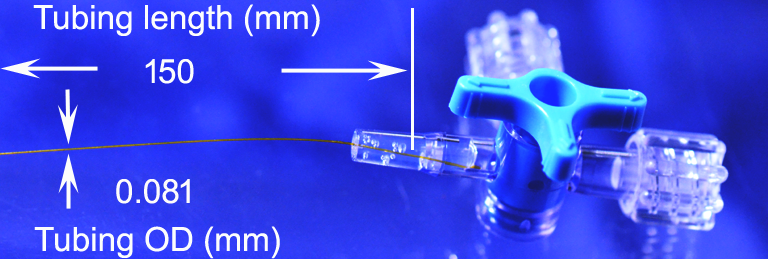
The above mentioned assembly problem is solved by Doccol. We proudly provide our Doccol microcatheters that have minimal inner volume and small outer diameter. The microcatheter comprises a single segment microtubing of uniform diameter connected to an industry standard Luer Taper connector or stopcock, with or without a guiding stylet. The catheter eliminates the unnecessary use of transitional tubing with stepwise increase of diameters for connecting to a Luer Taper connector, a stopcock, or a Luer needle; therefore, it reduces the inner volume of the catheter. The inner and outside diameter of the catheter tubular body can be as tiny as , but not limited to, 0.056 mm and 0.081 mm, respectively. For the microcatheters made of PI materials, the dead volume for catheters with FL connectors is 20-30 micro liter, for those with SC1 or SC4 connectors is 100-120 micro liter. Because the PI tubuings have very small I.D., the dead volume mainly comes from the connectors, where the space lies between the tubing inner end and the syringe tip. Because of greatly reduced inner volume, the catheter can be made as long as operational convenience requires.
References:
Cherkashova, E.A., Burunova, V.V., Bukharova, T.B. et al. Comparative Analysis of the Effects of Intravenous Administration of Placental Mesenchymal Stromal Cells and Neural Progenitor Cells Derived from Induced Pluripotent Cells on the Course of Acute Ischemic Stroke in Rats. Bull Exp Biol Med 166, 558–566 (2019) doi:10.1007/s10517-019-04392-5
Namestnikova, D & Gubskiy, Il'ya & Gabashvili, A & Sukhinich, Kirill & Melnikov, Pavel & Vishnevskiy, D & Soloveva, A & Vitushev, E & Chekhonin, V & Gubsky, Leonid & Yarygin, Konstantin. (2017). MRI evaluation of frequent complications after intra-arterial transplantation of mesenchymal stem cells in rats. Journal of Physics: Conference Series. 886. 012012. 10.1088/1742-6596/886/1/012012.
Namestnikova D, Gubskiy I, Kholodenko I, Melnikov P, Sukhinich K, et al. (2017) Methodological aspects of MRI of transplanted superparamagnetic iron oxide-labeled mesenchymal stem cells in live rat brain. PLOS ONE 12(10): e0186717. https://doi.org/10.1371/journal.pone.0186717
Arnberg F, Lundberg J, Kenne E, et al. Superselective intra-arterial umbilical cord blood administration to BM in experimental animals. Bone Marrow Transplantation. 2014;49(12):1486-1491. doi:10.1038/bmt.2014.190.
|

